A unique feature offered by go.Med with its medical device development services is the application of the Quality-by-Design (QbD) approach throughout the whole process.
QbD is a risk-based approach which introduces quality, efficacy and safety in the medical device development process from the start. If used properly, the QbD approach can help address issues early in the product development process & prevent late-stage development failures. 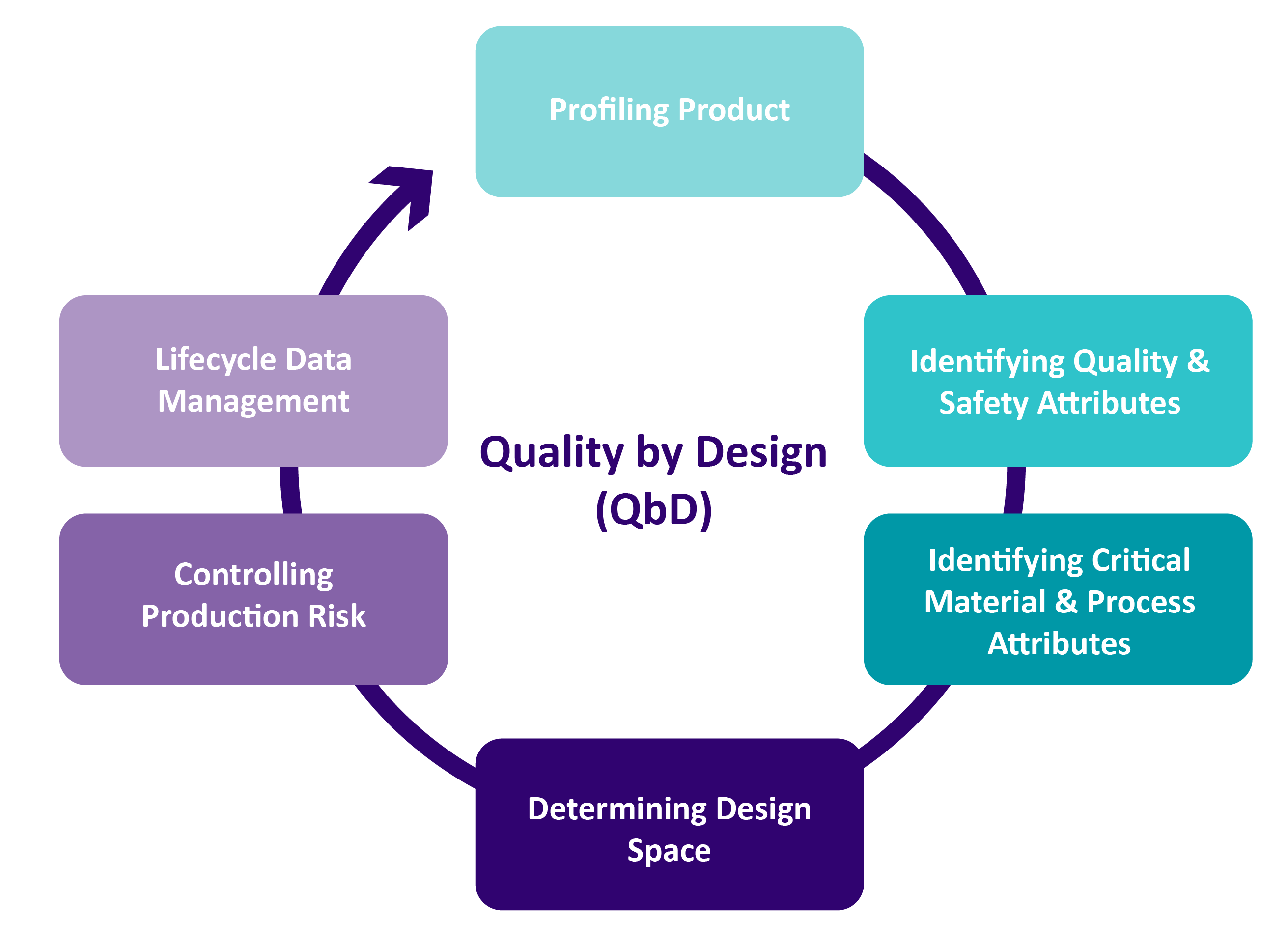
Applying QbD in the development process of medical devices will generate robust scientific knowledge based on a risk analysis that emphasizes product understanding and process control. This will lead to:
- Increasing the quality and reducing the risk of the medical device which will facilitate subsequent clinical testing
- Building arguments to demonstrate real benefits (such as value and final outcomes) of the new device to increase its success in entering the market and minimizing the time for reimbursement approval
- Reducing the cost and variability in the manufacturing process and increasing the speed of product release to the market by carrying out statistically designed experiments for process validation
The QbD approach is applied in the following phases of the medical device development:
- Concept Phase
- Development Phase
- Validation Phase
- Clinical Investigation Phase
If you are interested in learning more about the QbD approach, information is available here.