- 2nd HTA counselling session with clinicians after in vivo preclinical testing and a first risk assessment to identify the readiness of the device for clinical proof of concept and the necessary end-points to be considered.
- Design, management and execution of Clinical Proof of Concept (CPoC)
- Transfer of optimized manufacturing process to industrial production (ISO 13485 norm)
- 3rd HTA counselling session after CPoC and before clinical investigations (CI) to identify possible improvements to the protocol and the device.
- Design, management and execution of scaled-up (pivotal) clinical investigations (CI). According to the new EU Regulation 2017/745 Class IIb and Class III medical devices need to demonstrate safety and performance by clinical investigations
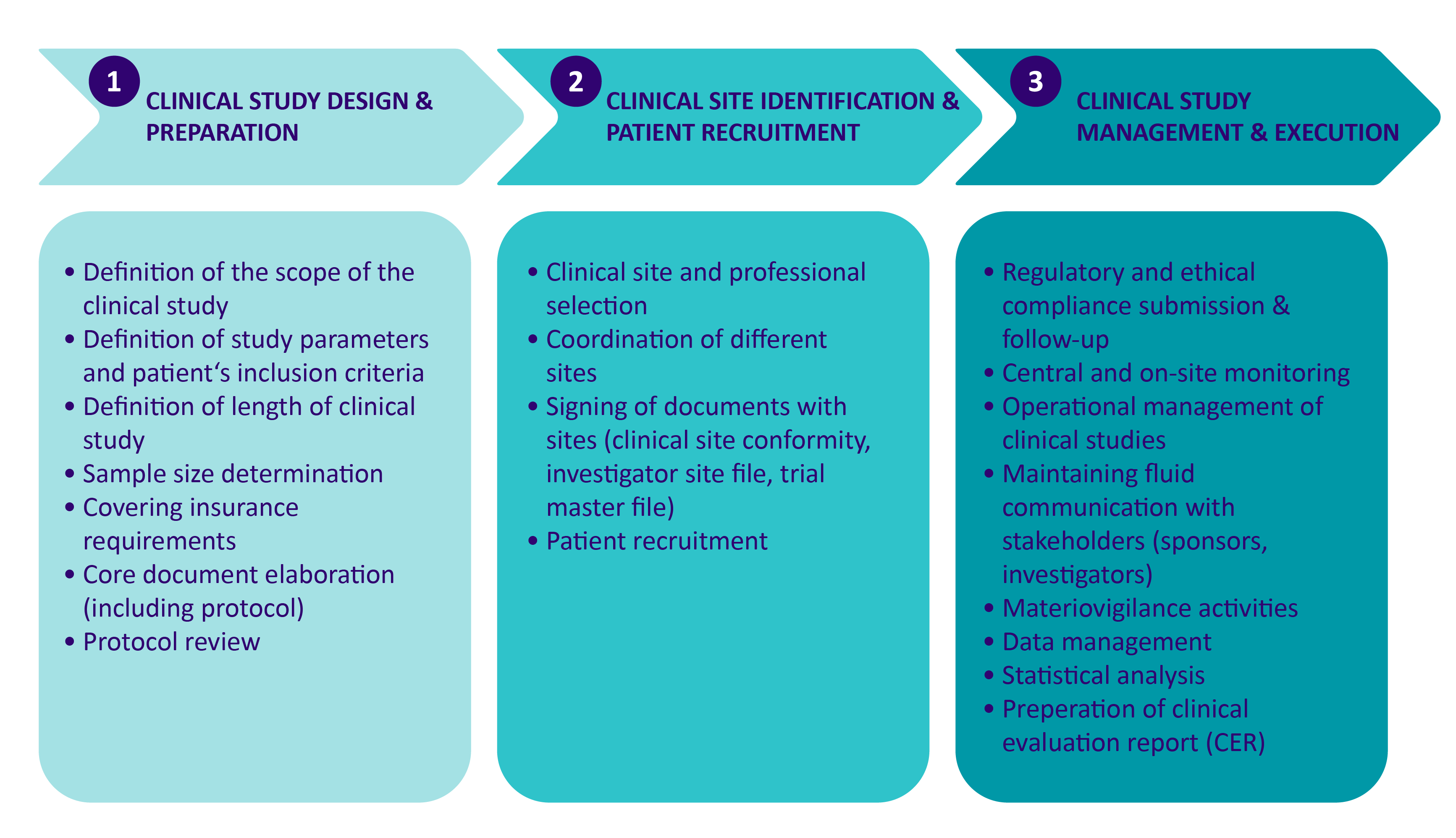
The Clinical Investigation phase can be divided in three different steps as in the figure below.